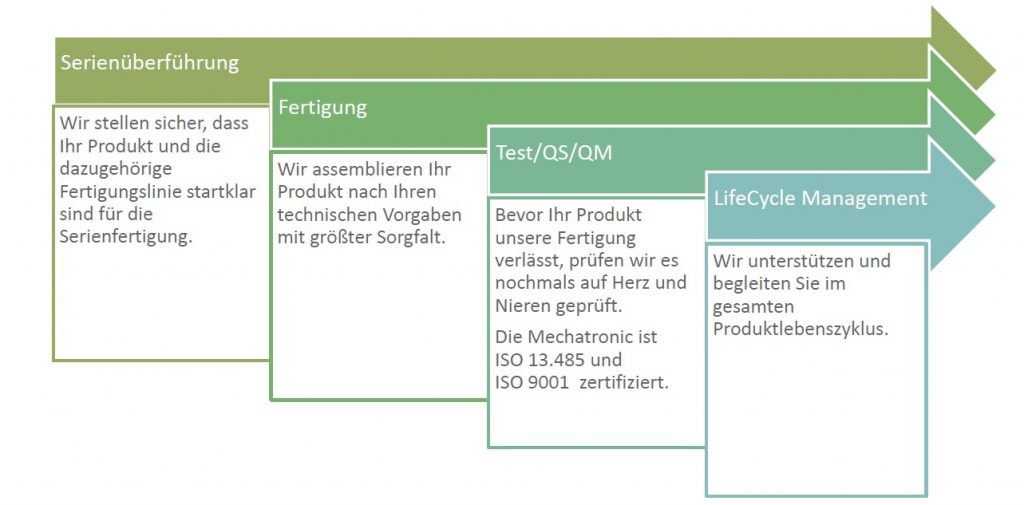
The manufacturing of small or medium numbers of medical and IVD devices meeting high regulatory and documentary requirements is no problem for the experts at Mechatronic.
We transfer the designed device directly into our manufacturing process and also smoothly transfer the whole manufacturing itself from a given location to ours.