Medical engineering means looking ahead even further. Here at Mechatronic, we are dealing with tomorrow today. The present state of development never lets us rest. It is the latest progress in medical treatment that sets the pace.
In order to handle a variety of projects successfully and achieve a high degree of customer satisfaction, we have to improve our methods constantly.
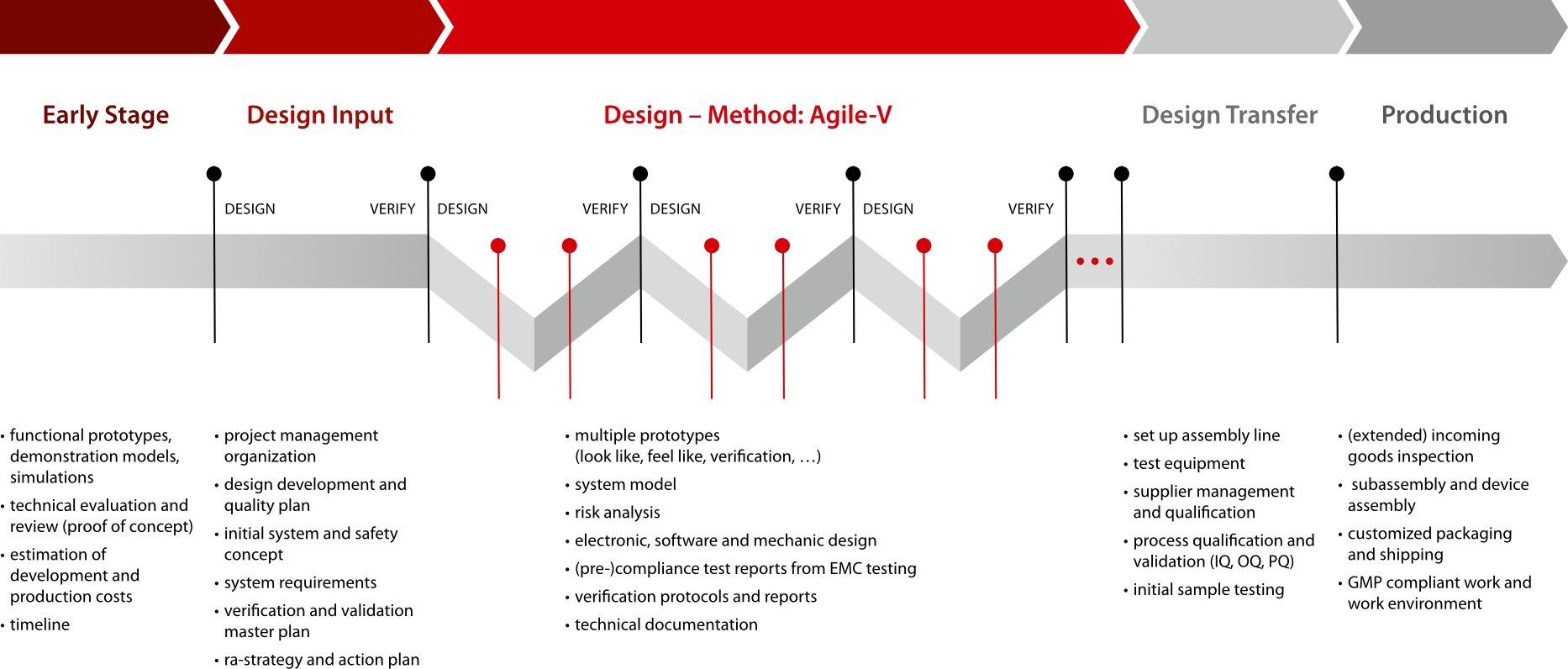
In every accomplished project we see a new learning opportunity. Based on thorough process analysis, we make further adjustments. This continuous improvement resulted in our “Agile V”.
Standard requirements, approved procedures based on the V-model as well as the agile approach derived from software development have been combined to create an innovative development process.
At the end of every project, our customers are entitled to receive a product which meets all regulatory requirements.