Bring your medical device manufacturing back to Germany now and benefit from “Made in Germany” manufacturing:
Is your ISO 13485 certified manufacturing affected by legal or political changes abroad?

- Short delivery routes
- No supply chain problems
- No time zones and language barriers
- No exchange rate risks
- Highest quality standards
- Crisis-proof location
- Specialized in series production in regulated manufacturing environment
- No negative effects due to legal and political changes abroad
- Easy access to our production at our manufacturing site in Höhn, Germany
- If desired, launch of your medical devices in Europe and the USA through our “Legal Manufacturer as a Service” offer.
China as the wrong location for manufacturing medical technology products
China has long since ceased to be the right location for the manufacture of medical devices in accordance with ISO 13485. At the latest since the Chinese government’s no-covid policy in recent years, it has become clear that manufacturing in China is associated with many risks – In addition to the difficulties caused by lock downs in the country, the supply routes to and from China have also become more vulnerable than was conceivable just a few years ago. In early 2021, for example, a single container ship in the Suez Canal managed to paralyze a major part of the global shipping trade. Was the production of your medical technology products also affected? – If not, you are one of the exceptions. That’s why we now offer “Made in Germany” manufacturing at the same price as offshore.

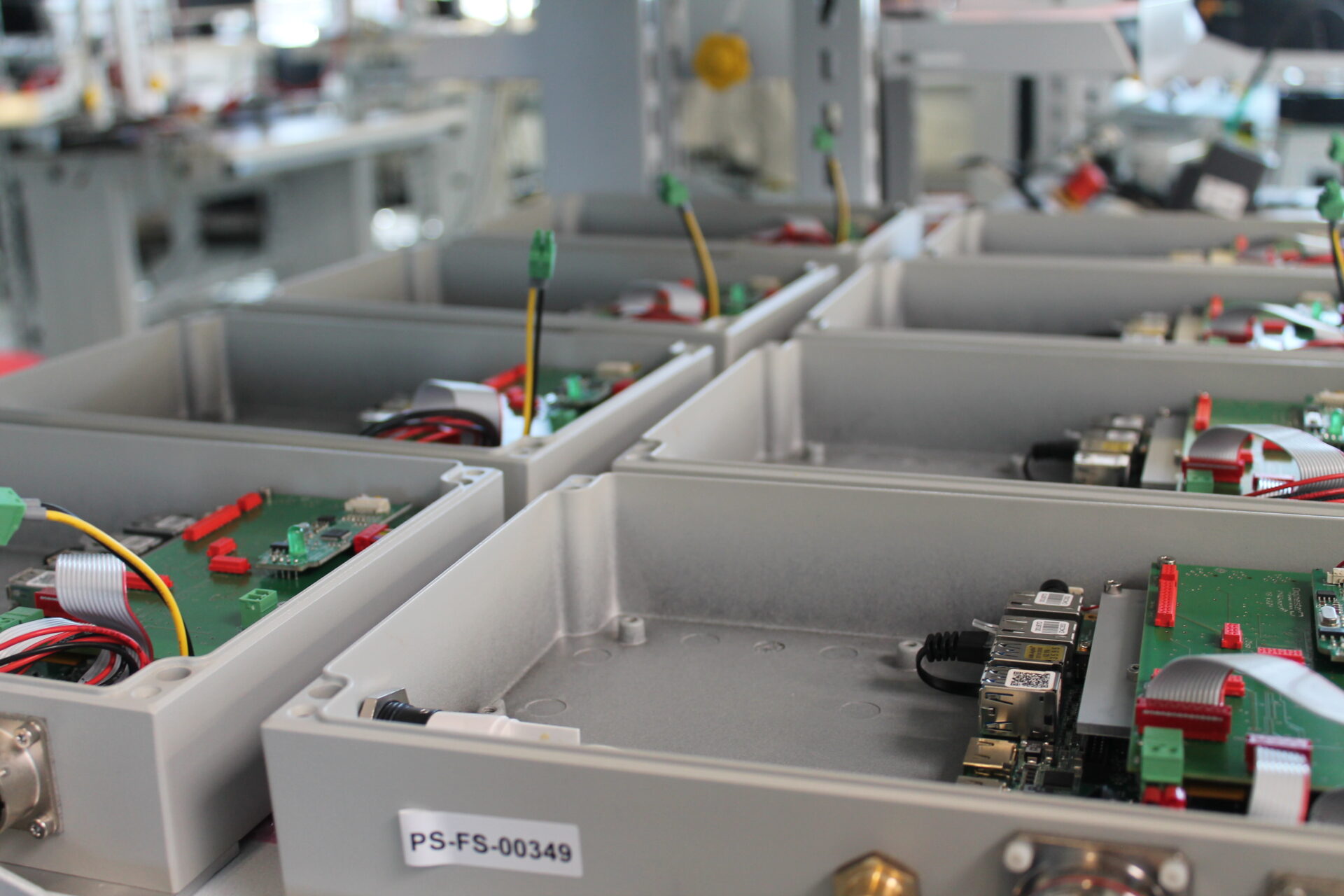

What is important for manufacturing according to ISO 13485?
The production of medical technology and IVD devices is carried out according to ISO 13485. High regulatory and documentary requirements in the production of medical and IVD devices are for our production teams no challenge. Under ideal conditions, we manufacture and test our customers’ medical technology products in small or medium quantities on more than 1000m2. All requirements from the standards ISO 13485, ISO 9001, FDA, CSA, CCC, Anvisa, UL and Vds are established in our house. From there we deliver the finished medical devices and IVD products all over the world.
ISO 13485 Manufacturing and ERP?
With an integrated ERP solution, we seamlessly track the manufacturing processes according to ISO 13485 for scheduling, procurement, production and delivery. We store the verification documents digitally for our customers in a document-safe manner (FDA and ISO 13485 compliant).
Market launch of medical devices – What needs to be considered?
We pay particular attention to the smooth launch of medical products. To avoid delays, we plan product and manufacturing development in parallel so that the source of errors during the transfer from development to manufacturing is minimized. Since the market launch of medical devices is a very cost-effective project, our colleagues at BAYOOCARE will be involved from the beginning of the project at your request. Our lawyers ensure that the regulatory aspects run smoothly. Completely carefree, because we know that every lost month during the market launch costs valuable market shares.



Medical device approval
The experts at BAYOOCARE GmbH, with offices in Germany, Switzerland and England, are our professionals when it comes to the approval of medical devices. So far, every medical device that BAYOOCARE’s lawyers have accompanied has been approved. The service for the approval of medical devices is easy for you to commission via our colleagues at Mechatronic, as we belong to the same group of companies. The advantage for you is that you only have to deal with one contact person, regardless of whether it is a matter of ISO 13485-compliant production, the market launch or the approval of your medical devices.
ISO 13485 manufacturing at offshore price
With more than 40 years of experience in the manufacturing of medical devices and the continuous improvement of our manufacturing according to ISO 13485, we can offer you “Made in Germany” manufacturing at an offshore price. You benefit from all the advantages of Germany as a business location, but do not pay more than for your production in e.g. China.

Güzel-Duygu Demir
sales manager

Karl Wörtche
head of key account management