Development of a continual in-line blood analysis monitor for the monitoring of vital blood parameters during extracorporeal circulation in heart surgery.

Portfolio: Maquet GmbH
TASK
CHALLENGES IN DEVELOPMENT
- Development from scratch (not a follow-up product)
-
Project start including feasibility studyCompilation of the initial product requirements and evaluation of the concepts in workshops with the customer
- Early assembly of a function model (also for usability engineering)
Integration project - Cooperation and coordination of both sensor development partners
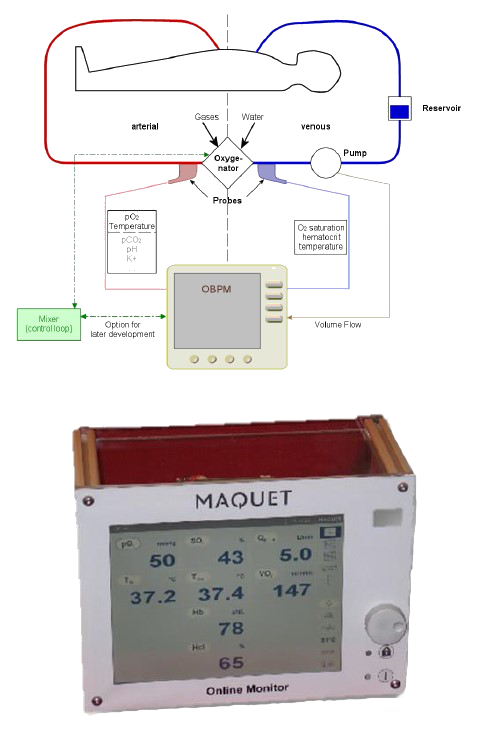

ALL INFORMATION AT A GLANCE
- Test units for production
- Duration of project to delivery of the first series device (manufactured at Mechatronic): 24 months
- Disciplines
– Mechanics: thermoformed plastic case, metal chassis (table and mast-mount)
– Electronic engineering: micro controller and embedded PC
– Software: C++ and Windows XP (GUI) (Interfaces: USB / USB Host / 2 x RS232) - Size of team: Nine developers on average, in project peak times fifteen developers
- Life Cycle Management (LCM)
– Change Management
– Obsolescence Component Management
– Second Level Service
– Re-design (3rd edition, 4th edition, additional parameters)
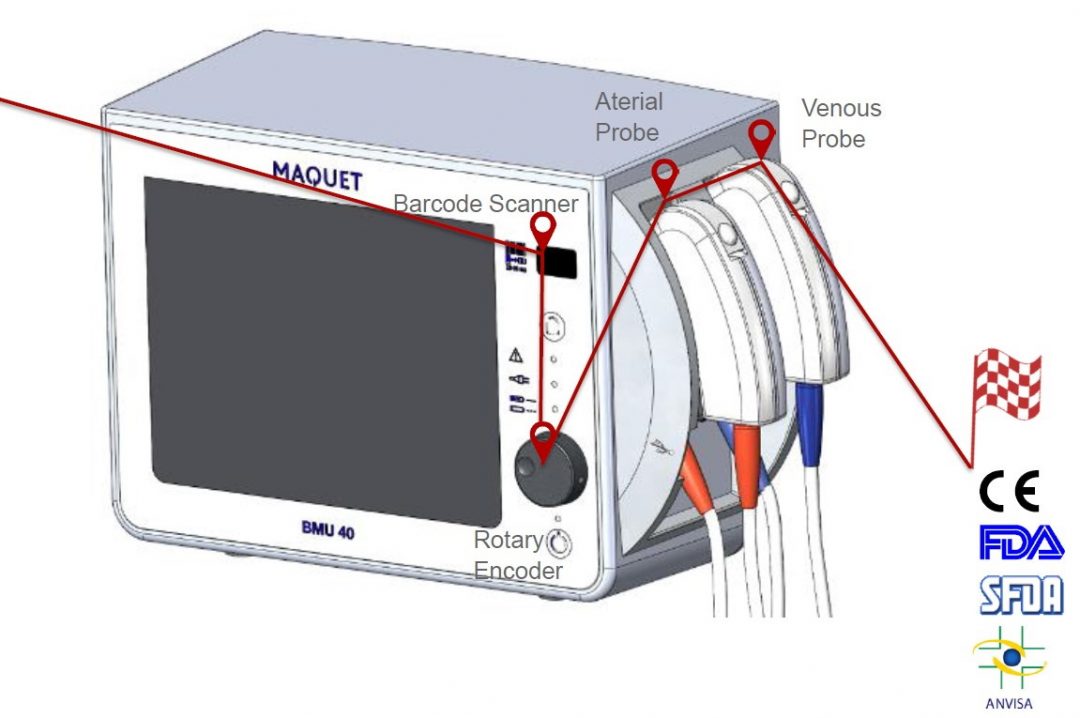
DEVICE INFORMATION
- Measures on display
– Arterial partial oxygen pressure (pO2)
– Arterial temperature
– Venous oxygen saturation (O2Sat)
– Venous hematocrit (Hct)
– Venous hemoglobin (Hb)
– Venous temperature - Site of operation
– Patient near-field
– OP - Interfaces (among others)
– Printer
– USB-Stick